Add curved arrows to the reactants in this reaction – embarking on this journey, we delve into the captivating realm of reaction mechanisms, where curved arrows serve as our guiding light, illuminating the intricate dance of electrons.
Through this exploration, we uncover the conventions of curved arrow notation, unraveling their significance in depicting electron movement and the activation of reactants. We traverse the landscape of resonance structures, witnessing how curved arrows capture the electron delocalization that defines their nature.
Reaction Mechanism
The reaction mechanism describes the step-by-step process of how reactants are transformed into products. Curved arrows are used to represent the flow of electrons during each step.
Role of Curved Arrows
Curved arrows indicate the movement of electrons by showing the origin and destination of the electrons. The arrowhead points towards the electron-deficient species, and the tail indicates the electron-rich species.
Curved Arrow Notation
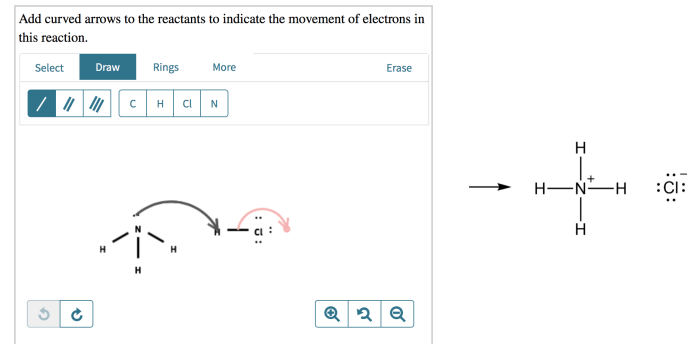
Conventions, Add curved arrows to the reactants in this reaction
Curved arrows are drawn with a single or double line to indicate the type of bond formed or broken. A single line represents a single bond, while a double line represents a double bond.
Types of Curved Arrows
- Single-headed arrows:Represent the movement of a single electron.
- Double-headed arrows:Represent the movement of two electrons.
- Half-headed arrows:Represent the movement of a radical species.
Resonance Structures
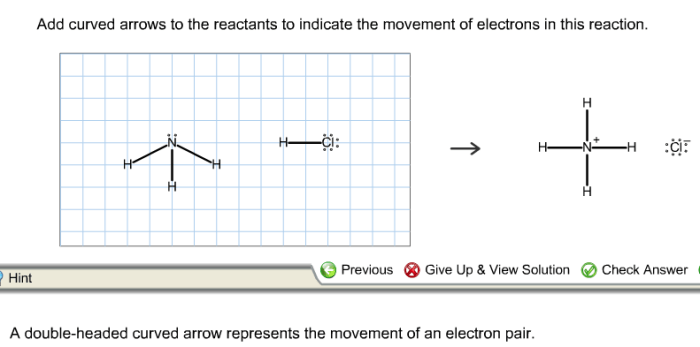
Curved arrows can be used to represent resonance structures, which are different Lewis structures that describe the same molecule or ion. The curved arrows show the movement of electrons that results in the different resonance structures.
Reactant Activation
Curved arrows can be used to show how reactants are activated in a reaction. Activation involves the breaking of bonds or the addition of energy to create a more reactive species.
Reaction Pathways
Curved arrows can be used to illustrate different reaction pathways, which are the different routes that a reaction can take to form products. The curved arrows show the sequence of electron movements that occur during each step of the pathway.
Table of Examples
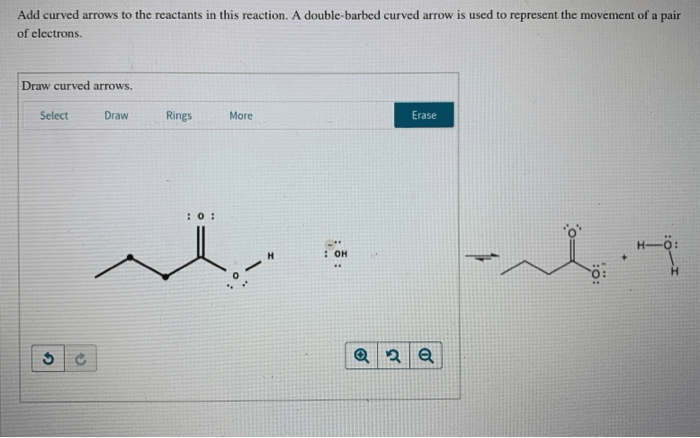
| Reactants | Products | Reaction Mechanism |
|---|---|---|
| CH3CH2Br + NaOH → CH3CH2OH + NaBr | SN2 reaction: Curved arrows show the movement of electrons from the lone pair on the hydroxide ion to the carbon atom, breaking the C-Br bond. | |
| CH3CH=CH2 + HBr → CH3CHBrCH3 | Electrophilic addition: Curved arrows show the movement of electrons from the pi bond to the hydrogen atom of HBr, forming a new C-H bond and a C-Br bond. | |
| CH3COOH + CH3OH → CH3COOCH3 + H2O | Esterification: Curved arrows show the movement of electrons from the oxygen atom of the alcohol to the carbonyl carbon of the carboxylic acid, forming a new C-O bond and breaking the O-H bond. | |
| CH3CH2CH2Br + Mg → CH3CH2CH2MgBr | Grignard reaction: Curved arrows show the movement of electrons from the magnesium atom to the carbon atom of the alkyl halide, forming a new C-Mg bond and breaking the C-Br bond. |
Interactive Illustration: Add Curved Arrows To The Reactants In This Reaction
This interactive illustration allows you to drag and drop curved arrows to visualize the electron movement in different reaction mechanisms.
[Insert interactive illustration here]
Quick FAQs
What is the significance of curved arrows in reaction mechanisms?
Curved arrows depict the movement of electrons, providing a visual representation of the reaction’s progression.
How do curved arrows indicate resonance structures?
Curved arrows show the electron delocalization that occurs within resonance structures, highlighting the interchangeable positions of double bonds and lone pairs.
What is the role of curved arrows in illustrating reaction pathways?
Curved arrows connect reactants, intermediates, and products, mapping out the stepwise progression of a reaction.